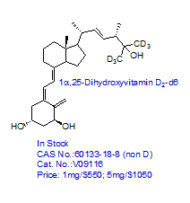
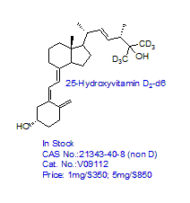
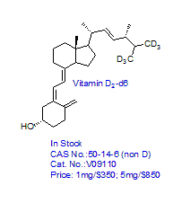
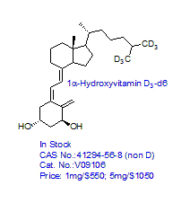
CRO Laboratories Inc. is a leading Contract Research Organization (CRO) headquartered in Dallas, Texas, providing end-to-end chemistry services to pharmaceutical, biotechnology, academic, and government organizations worldwide. Our core expertise includes synthetic chemistry, medicinal chemistry, custom synthesis, route scouting, process development, and process optimization, supporting drug discovery, preclinical development, scale-up, and commercialization programs.
CRO Laboratories Inc. is also a trusted global manufacturer and supplier of deuterium-labeled and ¹³C-labeled compounds, stable isotope-labeled drug substances, analytical reference standards, pharmaceutical standards, drug impurities, metabolites, active pharmaceutical ingredients (APIs), and specialty research chemicals. With over 4,000 in-stock catalog products and flexible production capabilities from milligram to kilogram scale, we deliver high-quality, reliable, fast-turnaround, and cost-effective solutions that accelerate research, regulatory filings, and commercial success.
Amines
Amino Acids
Analytical Reference Standards
Anti-Cancer Compounds
Anti-Diabetic Compounds
Anti-virals Compounds
Antibiotics
API Compounds
Azides / Acetylenes / Alkynes
BINOL & Derivatives
Biochemicals & Reagents
Biotinylation Reagents
Carbohydrates & Derivatives
Chiral Auxiliaries & Related
Dermatology Compounds
Deuterium-Labelled Compounds
Drug Discovery Reagents
Eicosanoids & Fatty acids
Fine Chemicals
Fluorescent Probes
Heterocyclic Compounds
Immunology Compounds
Impurities
Indoles & related
Inhibitors
Inositols & its phosphates
Intermediates
Microbiology Compounds
Neurochemicals
New Compounds
Nucleotides & Nucleosides
Peptides
Phospholipids
Screening Libraries
Steroids and Hormones
Vitamins & related
Contract Services
Medicinal Chemistry
- Design/synthesis of custom libraries
- Lead generation and optimization
- Synthesis of organic scaffolds
- Synthesis of pro-drugs and new chemical entities
Process Development
- Route design and optimization
- Process optimization and scale up to kg to multi-kg scale
- Large scale synthesis and purifications
- Production of pharmaceutical raw materials
Analytical Services
- Analytical and preparatory HPLC method development
- GC and LC-MS analysis
- Optical rotations and chiral separation
- Purity determinations by HPLC
Custom Synthesis
- Synthesis of intermediates
- Synthetic route development
- Metabolites synthesis/characterization
- Impurities isolation, synthesis and characterization
Scale-Up
- Scale-up from gm to kg non-GMP
- Scale-up from gm to kg GMP
- Synthesis of organic scaffolds
- Synthesis of pro-drugs, new chemical entities and advanced intermediates for preclinical studies
GMP Manufacturing
- Multi-kg services of pharmaceutical intermediates, active pharmaceutical ingredients (APIs), new chemical entities (NCEs) as well as cGMP manufacturing of drug substances