Additional information
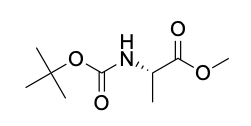
| Description | Boc-L-alanine-methyl-ester is a chemical compound used in peptide synthesis and organic chemistry. It is derived from L-alanine and features a tert-butyloxycarbonyl (Boc) protecting group on the amino group and a methyl ester group on the carboxylic acid. This compound is commonly employed as a building block in the synthesis of peptides and peptidomimetics due to its stability and compatibility with solid-phase peptide synthesis (SPPS) methodologies. The Boc protecting group shields the amino group from unwanted reactions during peptide chain assembly, while the methyl ester group serves as a temporary protection for the carboxylic acid, which can be easily removed under mild conditions. Boc-L-alanine-methyl-ester is a valuable tool in the field of medicinal chemistry and drug development, facilitating the creation of complex peptide structures for various biological studies and pharmaceutical applications. |
|---|---|
| Reference | 1) Methods of Synthesis of Remdesivir, Favipiravir, Hydroxychloroquine, and Chloroquine: Four Small Molecules Repurposed for Clinical Trials during the Covid-19 Pandemic |